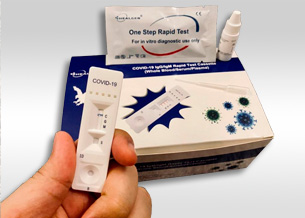
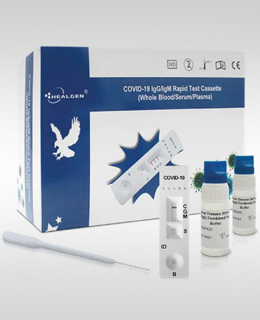
Healgen COVID-19 IgG/IgM Rapid Test Cassette
ANTIBODY DETECTION. MULTIVARIABLE ANALYSIS.The COVID-19 IgG/IgM (Whole Blood/Serum/Plasma) Rapid Test Device is very quick with a processing time of approximately 10 minutes.
The COVID-19 IgG/IgM is highly portable and does not require complex equipment.
DESCRIPTIOIN
The COVID-19 IgG/IgM (Whole Blood/Serum/Plasma) Rapid Test Device utilizes lateral flow technology that is used for the qualitative, differential detection of both anti-SARS-CoV-2 IgM and IgG antibodies. In general, antibodies can be detected 1-3 weeks after infection. This test is not intended to screen patients for active COVID-19 infection.
-
-
The sensitivity of the IgM test is 87.9% and specificity 100% when compared to RT-PCR.
The sensitivity of the IgG test is 97.2% during the convalescence period, and specificity is 100%.
- Positive results may be detected in as little as 10 minutes
- Detection Window (IgM): 3-5 days after incubation
- Dual band results for simple interpretation
- Multivariable analysis of immunoglobin IgG & IgM
- Room temperature storage or refrigerated (2-30⁰C / 36-86⁰F)
- Procedural internal control included
Fact Sheets
- Click here for Fact Sheet for Recipients
- Click her for Fact Sheet for Providers
Warning
This test has been authorized by FDA under an EUA for use by authorized laboratories. This test has not been FDA cleared or approved. This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. This product is intended for professional use and not for home use. Not for the screening of donated blood.
Note
- - The Healgen antibody test is not being promoted for Fingerstick use.
- - This product is not available to be purchased online from our website and is not sold “for at home use”.
SPECIFICATION
- Sensitivity: IgG 97.2%; IgM 87.9%
- Specificity: IgG 100%; IgM 100%
- DSpecimen: Whole Blood, Serum, Plasma
- Time to Results: 10 minutes
- Shelf Life: 24 months from the date of manufacture
Related Products
-
LOOKING FOR RESULTS?
Tiger Testing does not provide test results over the phone, email or tawk chat to individuals. If you were tested in Georgia or North Carolina and got a text from Tiger Testing (Radeas Labs) , please call 919-263-1150 or visit https://www.radeas.com/contact-us for test results. This company is not associated with us.


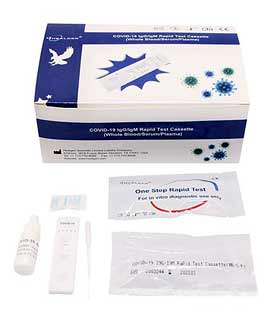





BENEFITS
- AntibodyDetection
- IgM Detection (Active Infection)
- IgG Detection (Previous Infection)
- Tiger Owned Asset
- Highly Portable
- Does not require complex equipment